How Many Unpaired Electrons Are Present in a Ground-state Atom From the Alkaline Earth Metal Family
three.i: Electron Configurations
- Folio ID
- 119829
Skills to Develop
- Derive the predicted footing-state electron configurations of atoms
- Identify and explain exceptions to predicted electron configurations for atoms and ions
- Predict the charge of common metallic and nonmetallic elements, and write their electron configurations
- Chronicle electron configurations to element classifications in the periodic tabular array
Having introduced the nuts of atomic construction and quantum mechanics, we can use our understanding of quantum numbers to decide how atomic orbitals relate to 1 another. This allows united states to decide which orbitals are occupied by electrons in each atom. The specific arrangement of electrons in orbitals of an atom determines many of the chemic properties of that atom.
Orbital Energies and Diminutive Structure
The free energy of diminutive orbitals increases as the principal quantum number, \(n\), increases. In any atom with two or more electrons, the repulsion betwixt the electrons makes energies of subshells with dissimilar values of \(l\) differ then that the energy of the orbitals increases within a shell in the order s < p < d < f. Figure \(\PageIndex{one}\) depicts how these 2 trends in increasing free energy relate. The onedue south orbital at the bottom of the diagram is the orbital with electrons of lowest free energy. The energy increases equally nosotros motility up to the 2s then 2p, 3southward, and threep orbitals, showing that the increasing due north value has more than influence on free energy than the increasing fifty value for minor atoms. However, this pattern does non hold for larger atoms. The 3d orbital is higher in energy than the 4s orbital. Such overlaps go along to occur oftentimes equally we movement up the chart.
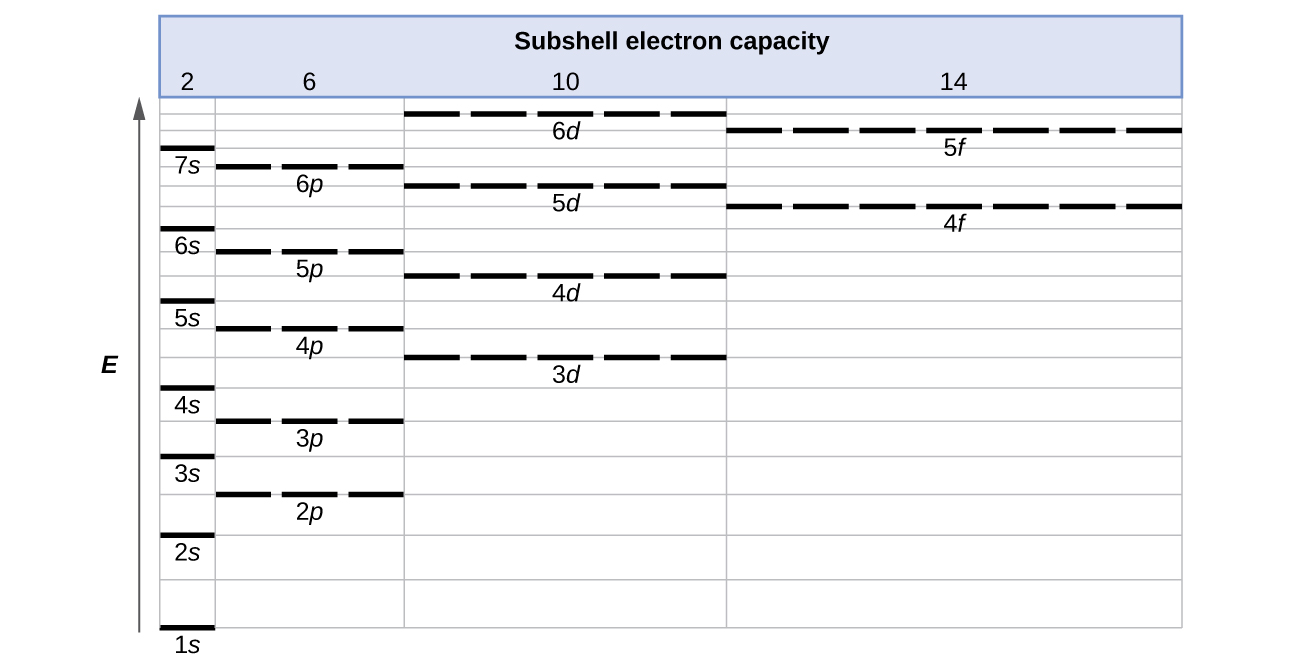
Figure \(\PageIndex{1}\) : Generalized energy-level diagram for diminutive orbitals in an cantlet with two or more electrons (not to scale).
Electrons in successive atoms on the periodic table tend to make full low-energy orbitals start. Thus, many students find it confusing that, for case, the vp orbitals make full immediately after the 4d, and immediately before the 6s. The filling order is based on observed experimental results, and has been confirmed by theoretical calculations. Equally the principal quantum number, n, increases, the size of the orbital increases and the electrons spend more than time farther from the nucleus. Thus, the attraction to the nucleus is weaker and the energy associated with the orbital is higher (less stabilized). But this is not the only effect we have to accept into account. Within each crush, as the value of l increases, the electrons are less penetrating (meaning there is less electron density found shut to the nucleus), in the order due south > p > d > f. Electrons that are closer to the nucleus slightly repel electrons that are further out, offsetting the more dominant electron–nucleus attractions slightly (recall that all electrons have −1 charges, simply nuclei have +Z charges). This miracle is called shielding and volition be discussed in more than item in the side by side section. Electrons in orbitals that experience more shielding are less stabilized and thus higher in energy. For small orbitals (is through 3p), the increase in energy due to n is more significant than the increase due to 50; however, for larger orbitals the 2 trends are comparable and cannot be simply predicted. Nosotros will discuss methods for remembering the observed order.
The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. We describe an electron configuration with a symbol that contains three pieces of information ( Figure \(\PageIndex{ii}\)):
- The number of the principal quantum shell, n,
- The alphabetic character that designates the orbital blazon (the subshell, 50), and
- A superscript number that designates the number of electrons in that detail subshell.
For example, the notation 2p iv (read "2–p–iv") indicates 4 electrons in a p subshell (l = 1) with a primary quantum number (n) of 2. The notation 3d eight (read "iii–d–eight") indicates eight electrons in the d subshell (i.e., l = 2) of the main trounce for which n = iii.
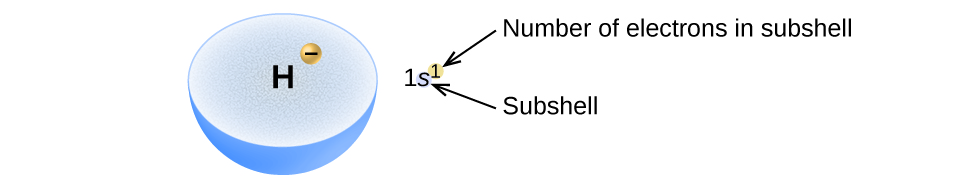
Figure \(\PageIndex{2}\) : The diagram of an electron configuration specifies the subshell (northward and l value, with letter of the alphabet symbol) and superscript number of electrons.
The Aufbau Principle
To determine the electron configuration for whatsoever detail atom, we can "build" the structures in the club of atomic numbers. Commencement with hydrogen, and continuing across the periods of the periodic table, we add one proton at a time to the nucleus and 1 electron to the proper subshell until nosotros accept described the electron configurations of all the elements. This procedure is chosen the Aufbau principle, from the High german word Aufbau ("to build upwards"). Each added electron occupies the subshell of everyman energy bachelor (in the order shown in Figure \(\PageIndex{3}\)), field of study to the limitations imposed past the allowed quantum numbers according to the Pauli exclusion principle. Electrons enter higher-energy subshells simply after lower-free energy subshells have been filled to capacity. Figure \(\PageIndex{3}\) illustrates the traditional way to remember the filling gild for diminutive orbitals.
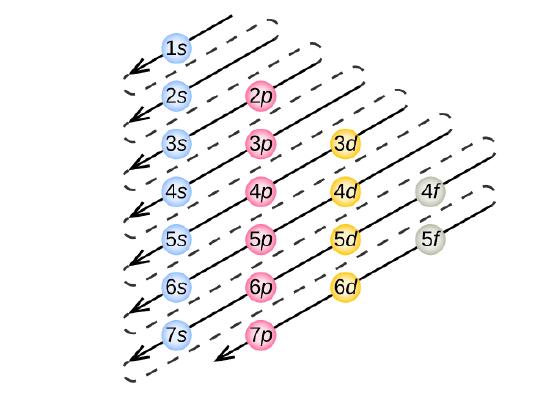
Figure \(\PageIndex{3}\) : The arrow leads through each subshell in the appropriate filling order for electron configurations. This chart is straightforward to construct. Just brand a cavalcade for all the s orbitals with each n shell on a separate row. Repeat for p, d, and f. Exist sure to only include orbitals allowed by the quantum numbers (no 1p or 2d, and and so forth). Finally, draw diagonal lines from top to bottom equally shown.
Since the arrangement of the periodic tabular array is based on the electron configurations, Figure \(\PageIndex{4}\) provides an culling method for determining the electron configuration. The filling club just begins at hydrogen and includes each subshell equally you proceed in increasing Z order. For example, after filling the 3p block upwardly to Ar, we see the orbital will be 4s (M, Ca), followed by the 3d orbitals.
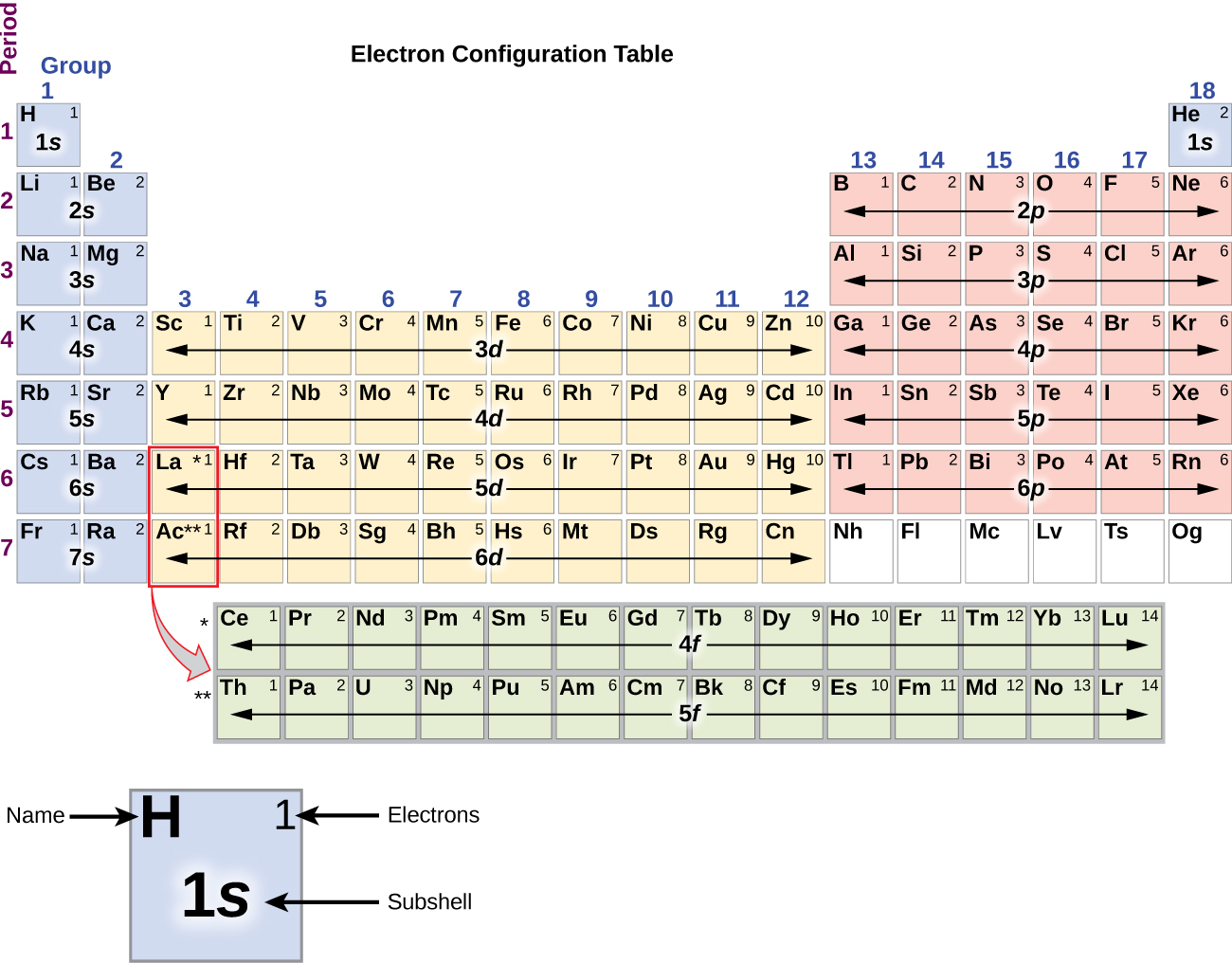
Figure \(\PageIndex{4}\): This periodic table shows the electron configuration for each subshell. Past "edifice up" from hydrogen, this tabular array can be used to make up one's mind the electron configuration for whatever atom on the periodic table.
We volition now construct the basis-state electron configuration and orbital diagram for a selection of atoms in the kickoff and second periods of the periodic table. Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of electrons. We get-go with a unmarried hydrogen atom (diminutive number i), which consists of one proton and one electron. Referring to either Figure \(\PageIndex{3}\) or \(\PageIndex{4}\), we would expect to find the electron in the 1south orbital. By convention, the
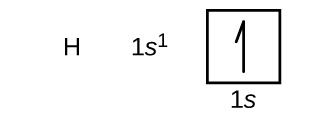
Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron (n = 1, l = 0, ml = 0, \(m_s=+\dfrac{1}{ii}\)
The n = 1 shell is completely filled in a helium atom.
The next atom is the alkaline metal lithium with an atomic number of iii. The first two electrons in lithium fill up the 1due south orbital and accept the aforementioned sets of four quantum numbers equally the two electrons in helium. The remaining electron must occupy the orbital of side by side lowest energy, the 2s orbital ( Effigy \(\PageIndex{iii}\) or \(\PageIndex{four}\) ). Thus, the electron configuration and orbital diagram of lithium are:
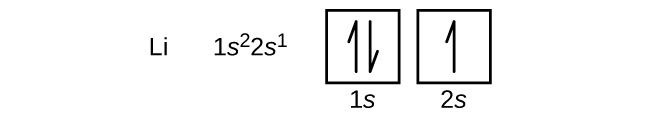
An atom of the alkaline globe metal beryllium, with an atomic number of 4, contains four protons in the nucleus and 4 electrons surrounding the nucleus. The fourth electron fills the remaining space in the iis orbital.
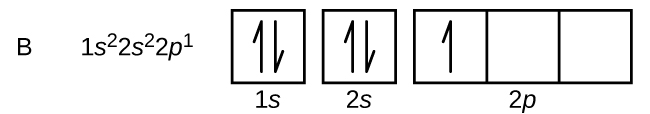
An atom of boron (atomic number 5) contains five electrons. The northward = one shell is filled with two electrons and three electrons volition occupy the n = 2 shell. Because whatsoever due south subshell tin can contain merely two electrons, the fifth electron must occupy the adjacent energy level, which will be a 2p orbital. There are iii degenerate 2p orbitals (gfifty = −1, 0, +1) and the electron can occupy any one of these p orbitals. When drawing orbital diagrams, we include empty boxes to draw whatever empty orbitals in the same subshell that we are filling.
Carbon (atomic number half-dozen) has vi electrons. Four of them fill the 1s and 2s orbitals. The remaining two electrons occupy the 2p subshell. We at present take a choice of filling i of the iip orbitals and pairing the electrons or of leaving the electrons unpaired in 2 different, but degenerate, p orbitals. The orbitals are filled as described by Hund'south rule: the lowest-energy configuration for an atom with electrons inside a set up of degenerate orbitals is that having the maximum number of unpaired electrons. Thus, the two electrons in the carbon twop orbitals have identical n, l, and msouthward quantum numbers and differ in their yardl quantum number (in accord with the Pauli exclusion principle). The electron configuration and orbital diagram for carbon are:
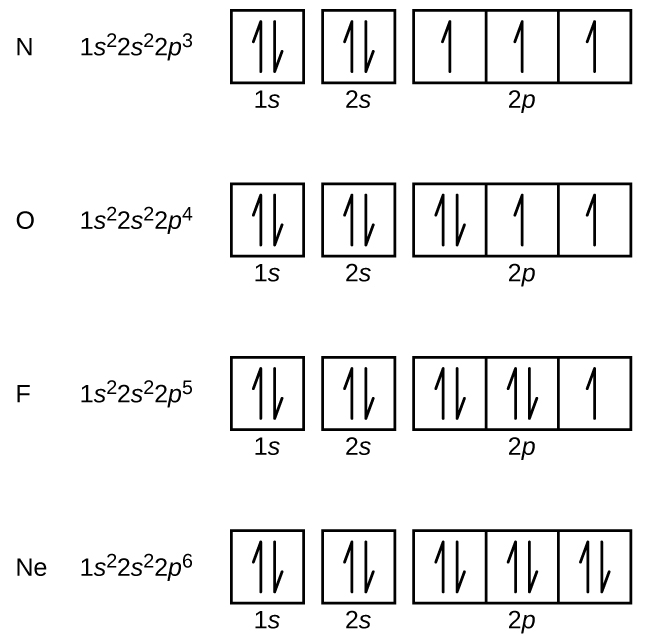
Nitrogen (atomic number 7) fills the anes and 2s subshells and has one electron in each of the three 2p orbitals, in accord with Hund'due south rule. These three electrons take unpaired spins. Oxygen (atomic number 8) has a pair of electrons in whatsoever i of the 2p orbitals (the electrons have opposite spins) and a single electron in each of the other two. Fluorine (atomic number ix) has simply ane twop orbital containing an unpaired electron. All of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled. The electron configurations and orbital diagrams of these four elements are:
The alkali metal sodium (atomic number 11) has one more electron than the neon cantlet. This electron must go into the lowest-energy subshell available, the 3s orbital, giving a 1s iitwos ii2p half dozen3s 1 configuration. The electrons occupying the outermost shell orbital(due south) (highest value of n) are called valence electrons, and those occupying the inner beat out orbitals are called core electrons ( Figure \(\PageIndex{5}\)). Since the core electron shells stand for to noble gas electron configurations, we tin abbreviate electron configurations past writing the element of group 0 that matches the cadre electron configuration, along with the valence electrons in a condensed format. For our sodium example, the symbol [Ne] represents core electrons, (1due south ii2southward two2p 6) and our abbreviated or condensed configuration is [Ne]3due south 1.

Figure \(\PageIndex{v}\) : A core-abbreviated electron configuration (right) replaces the cadre electrons with the element of group 0 symbol whose configuration matches the cadre electron configuration of the other element.
Similarly, the abbreviated configuration of lithium can exist represented as [He]2s 1, where [He] represents the configuration of the helium atom, which is identical to that of the filled inner shell of lithium. Writing the configurations in this mode emphasizes the similarity of the configurations of lithium and sodium. Both atoms, which are in the alkali metal family, have only one electron in a valence s subshell outside a filled set of inner shells.
\[\ce{Li:[He]}\,2s^1\\ \ce{Na:[Ne]}\,3s^1\]
The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a [Ne]3southward 2 configuration, is analogous to its family member glucinium, [He]2due south two. Both atoms have a filled south subshell outside their filled inner shells. Aluminum (atomic number 13), with xiii electrons and the electron configuration [Ne]3s 23p 1, is coordinating to its family member boron, [He]2southward 22p 1.
The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (sixteen electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their respective family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the master breakthrough number of the outer crush of the heavier elements has increased by one to due north = 3. Figure \(\PageIndex{6}\) shows the lowest energy, or footing-state, electron configuration for these elements equally well every bit that for atoms of each of the known elements.
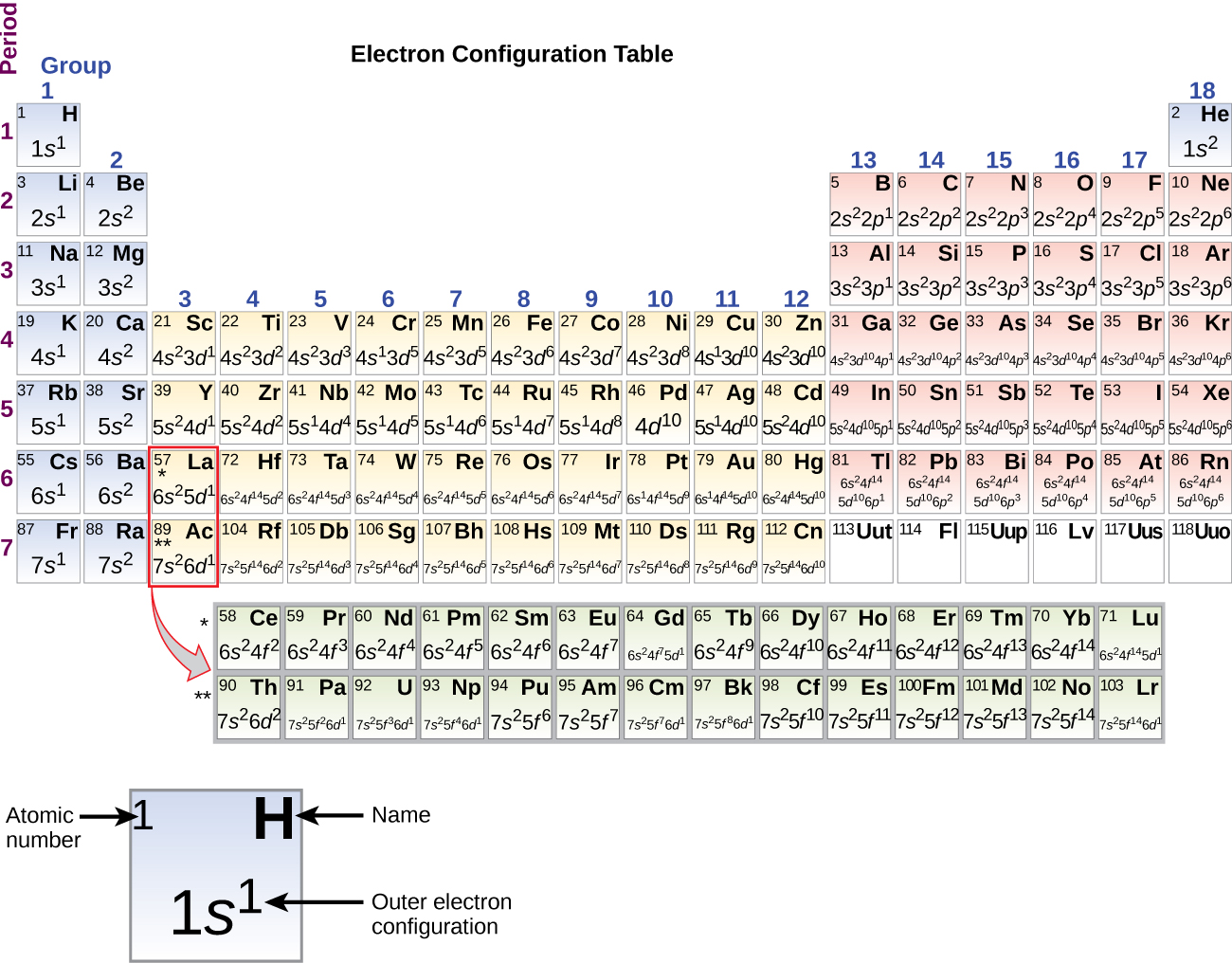
Figure \(\PageIndex{6}\) : This version of the periodic table shows the outer-shell electron configuration of each chemical element. Note that down each group, the configuration is often similar.
When we come to the next element in the periodic table, the brine metal potassium (diminutive number xix), we might expect that we would begin to add electrons to the iiid subshell. Yet, all available chemical and physical evidence indicates that potassium is like lithium and sodium, and that the next electron is non added to the 3d level just is, instead, added to the ivs level (Figure \(\PageIndex{iii}\) or \(\PageIndex{4}\)). Equally discussed previously, the iiid orbital with no radial nodes is college in energy because it is less penetrating and more shielded from the nucleus than the ivs, which has three radial nodes. Thus, potassium has an electron configuration of [Ar]fours 1. Hence, potassium corresponds to Li and Na in its valence vanquish configuration. The next electron is added to consummate the 4s subshell and calcium has an electron configuration of [Ar]4south 2. This gives calcium an outer-shell electron configuration corresponding to that of beryllium and magnesium.
Commencement with the transition metallic scandium (atomic number 21), additional electrons are added successively to the 3d subshell. This subshell is filled to its capacity with 10 electrons (call back that for l = 2 [d orbitals], at that place are 2l + 1 = 5 values of thousandl , meaning that there are five d orbitals that have a combined capacity of 10 electrons). The 4p subshell fills next. Note that for iii series of elements, scandium (Sc) through copper (Cu), yttrium (Y) through silver (Ag), and lutetium (Lu) through gold (Au), a total of 10 d electrons are successively added to the (n – one) shell adjacent to the n shell to bring that (due north – one) shell from 8 to xviii electrons. For 2 series, lanthanum (La) through lutetium (Lu) and actinium (Air conditioning) through lawrencium (Lr), fourteen f electrons (l = three, 2l + 1 = 7 m50 values; thus, vii orbitals with a combined capacity of fourteen electrons) are successively added to the (n – 2) beat to bring that vanquish from 18 electrons to a total of 32 electrons.
Example \(\PageIndex{ane}\): Quantum Numbers and Electron Configurations
What is the electron configuration and orbital diagram for a phosphorus cantlet? What are the four quantum numbers for the last electron added?
Solution
The atomic number of phosphorus is 15. Thus, a phosphorus atom contains 15 electrons. The order of filling of the energy levels is is, 2s, 2p, 3south, 3p, 4s, . . . The 15 electrons of the phosphorus atom volition fill up to the iiip orbital, which volition comprise 3 electrons:
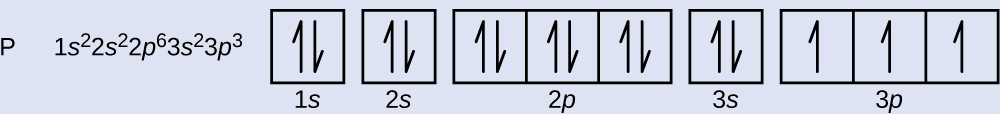
The last electron added is a 3p electron. Therefore, n = iii and, for a p-type orbital, l = 1. The kl value could be –1, 0, or +i. The 3 p orbitals are degenerate, so any of these m50 values is right. For unpaired electrons, convention assigns the value of
Practise \(\PageIndex{1}\)
Place the atoms from the electron configurations given:
- [Ar]4south two3d 5
- [Kr]5southward 24d 10fivep half-dozen
- Answer a
-
Mn
- Answer b
-
Xe
The periodic table can be a powerful tool in predicting the electron configuration of an chemical element. However, we exercise discover exceptions to the gild of filling of orbitals that are shown in Figure \(\PageIndex{3}\) or \(\PageIndex{4}\). For instance, the electron configurations of the transition metals chromium (Cr; diminutive number 24) and copper (Cu; atomic number 29), amid others, are not those we would wait. In general, such exceptions involve subshells with very similar energy, and small furnishings can lead to changes in the gild of filling.
In the instance of Cr and Cu, we find that half-filled and completely filled subshells evidently represent conditions of preferred stability. This stability is such that an electron shifts from the 4s into the iiid orbital to gain the extra stability of a half-filled 3d subshell (in Cr) or a filled threed subshell (in Cu). Other exceptions likewise occur. For instance, niobium (Nb, atomic number 41) is predicted to have the electron configuration [Kr]5south 24d three. Experimentally, nosotros notice that its ground-country electron configuration is actually [Kr]fives 14d iv. We can rationalize this ascertainment by saying that the electron–electron repulsions experienced by pairing the electrons in the 5s orbital are larger than the gap in energy between the 5s and fourd orbitals. There is no simple method to predict the exceptions for atoms where the magnitude of the repulsions between electrons is greater than the pocket-size differences in energy between subshells.
Electron Configurations and the Periodic Table
Video \(\PageIndex{1}\) : A trick for writing electron configurations based on the organization of the periodic table.
As described earlier, the periodic table arranges atoms based on increasing atomic number so that elements with the aforementioned chemical backdrop recur periodically. When their electron configurations are added to the tabular array (Figure \(\PageIndex{vi}\)), we also see a periodic recurrence of similar electron configurations in the outer shells of these elements. Considering they are in the outer shells of an atom, valence electrons play the nigh important role in chemical reactions. The outer electrons accept the highest free energy of the electrons in an cantlet and are more easily lost or shared than the cadre electrons. Valence electrons are also the determining factor in some physical properties of the elements.
Elements in any 1 group (or cavalcade) have the same number of valence electrons; the brine metals lithium and sodium each accept simply one valence electron, the alkaline earth metals beryllium and magnesium each take two, and the halogens fluorine and chlorine each have seven valence electrons. The similarity in chemical properties amid elements of the same group occurs because they accept the aforementioned number of valence electrons. Information technology is the loss, gain, or sharing of valence electrons that defines how elements react.
Information technology is important to remember that the periodic table was developed on the basis of the chemic behavior of the elements, well earlier whatever idea of their atomic construction was available. Now nosotros can empathise why the periodic table has the arrangement information technology has—the arrangement puts elements whose atoms have the same number of valence electrons in the same grouping. This arrangement is emphasized in Figure \(\PageIndex{half-dozen}\), which shows in periodic-tabular array class the electron configuration of the last subshell to be filled past the Aufbau principle. The colored sections of Figure \(\PageIndex{six}\) show the three categories of elements classified by the orbitals existence filled: principal group, transition, and inner transition elements. These classifications make up one's mind which orbitals are counted in the valence shell, or highest energy level orbitals of an atom.
- Primary group elements (sometimes called representative elements) are those in which the last electron added enters an due south or a p orbital in the outermost shell, shown in bluish and ruby-red in Figure \(\PageIndex{6}\). This category includes all the nonmetallic elements, as well every bit many metals and the intermediate semimetallic elements. The valence electrons for main group elements are those with the highest n level. For case, gallium (Ga, atomic number 31) has the electron configuration [Ar]4s ii 3d 10 4p 1 , which contains three valence electrons (underlined). The completely filled d orbitals count every bit cadre, not valence, electrons.
- Transition elements or transition metals. These are metal elements in which the final electron added enters a d orbital. The valence electrons (those added after the last noble gas configuration) in these elements include the ns and (due north – 1) d electrons. The official IUPAC definition of transition elements specifies those with partially filled d orbitals. Thus, the elements with completely filled orbitals (Zn, Cd, Hg, every bit well equally Cu, Ag, and Au in Figure \(\PageIndex{6}\)) are not technically transition elements. However, the term is often used to refer to the entire d block (colored yellow in Effigy \(\PageIndex{6}\)), and we will adopt this usage in this textbook.
- Inner transition elements are metallic elements in which the last electron added occupies an f orbital. They are shown in green in Figure \(\PageIndex{half-dozen}\). The valence shells of the inner transition elements consist of the (n – 2)f, the (n – i)d, and the ns subshells. There are two inner transition series:
- The lanthanide series: lanthanide (La) through lutetium (Lu)
- The actinide serial: actinide (Ac) through lawrencium (Lr)
Lanthanum and actinium, because of their similarities to the other members of the serial, are included and used to name the series, even though they are transition metals with no f electrons.
Electron Configurations of Ions
We have seen that ions are formed when atoms gain or lose electrons. A cation (positively charged ion) forms when one or more electrons are removed from a parent atom. For primary group elements, the electrons that were added concluding are the beginning electrons removed. For transition metals and inner transition metals, however, electrons in the s orbital are easier to remove than the d or f electrons, so the highest ns electrons are lost, and then the (n – 1)d or (north – 2)f electrons are removed. An anion (negatively charged ion) forms when one or more electrons are added to a parent atom. The added electrons fill up in the club predicted by the Aufbau principle.
Example \(\PageIndex{ii}\): Predicting Electron Configurations of Ions
What is the electron configuration and orbital diagram of:
- Na+
- Pthree–
- Altwo+
- Feii+
- Sm3+
Solution
Kickoff, write out the electron configuration for each parent atom. We have chosen to prove the full, unabbreviated configurations to provide more practice for students who want it, simply listing the core-abbreviated electron configurations is also acceptable.
Next, determine whether an electron is gained or lost. Remember electrons are negatively charged, so ions with a positive charge have lost an electron. For master group elements, the final orbital gains or loses the electron. For transition metals, the terminal s orbital loses an electron before the d orbitals.
- Na: idue south 2twosouthward 22p 6iiis ane. Sodium cation loses i electron, so Na+: 1due south ii2south 22p 63s 1 = Na+: 1south ii2s iitwop 6.
- P: idue south two2s 2iip 63southward 2iiip iii. Phosphorus trianion gains iii electrons, so Piii−: ones 22south ii2p 63southward 23p 6.
- Al: 1s 22s ii2p 63s 23p 1. Aluminum dication loses two electrons Al2+: anes two2s 2iip 6threes 23p 1 = Al2+: anes 22south two2p 6iiisouthward 1.
- Fe: 1due south twotwos 22p vi3southward 23p 64s 23d 6. Atomic number 26(II) loses two electrons and, since information technology is a transition metal, they are removed from the ivs orbital Fe2+: 1s 2iis two2p 6threes 2iiip six4s 23d 6 = 1due south ii2s 2twop 63s two3p 63d 6.
- Sm: onedue south iitwos 22p six3s 2iiip 64southward two3d 104p vi5south iiivd 105p 66due south two4f 6. Samarium trication loses three electrons. The first ii will be lost from the half dozendue south orbital, and the last one is removed from the 4f orbital. Sm3+: 1s 22south 22p six3southward two3p six4s 23d 10ivp half dozen5s 24d 105p 6vis 24f 6 = is 2iisouthward 22p half-dozen3s 2iiip sixfoursouthward two3d 104p half-dozen5southward 2fourd 105p 64f five.
Practice \(\PageIndex{ii}\)
- Which ion with a +2 accuse has the electron configuration 1s ii2s 22p six3s 23p 6threed 104s 2ivp 64d five?
- Which ion with a +three charge has this configuration?
- Respond a
-
Tc2+
- Answer b
-
Ru3+
Electronic Structures of Cations
When forming a cation, an atom of a main group chemical element tends to lose all of its valence electrons, thus bold the electronic construction of the element of group 0 that precedes it in the periodic table. For groups 1 (the alkali metals) and ii (the alkaline world metals), the group numbers are equal to the numbers of valence shell electrons and, consequently, to the charges of the cations formed from atoms of these elements when all valence shell electrons are removed. For example, calcium is a group ii element whose neutral atoms take 20 electrons and a footing state electron configuration of 1s ii2s ii2p 63s 23p half dozenfoursouth 2. When a Ca atom loses both of its valence electrons, the result is a cation with eighteen electrons, a 2+ charge, and an electron configuration of 1s 2iidue south 22p 63s 2threep half dozen. The Ca2+ ion is therefore isoelectronic with the noble gas Ar.
For groups 12–17, the group numbers exceed the number of valence electrons by 10 (accounting for the possibility of total d subshells in atoms of elements in the fourth and greater periods). Thus, the charge of a cation formed by the loss of all valence electrons is equal to the group number minus 10. For case, aluminum (in group 13) forms 3+ ions (Al3+).
Exceptions to the expected behavior involve elements toward the bottom of the groups. In addition to the expected ions Tl3+, Sn4+, Pb4+, and Bi5+, a partial loss of these atoms' valence shell electrons can also lead to the germination of Tl+, Sntwo+, Pb2+, and Bi3+ ions. The formation of these ane+, 2+, and three+ cations is ascribed to the inert pair effect, which reflects the relatively low energy of the valence southward-electron pair for atoms of the heavy elements of groups xiii, 14, and 15. Mercury (group 12) also exhibits an unexpected beliefs: it forms a diatomic ion, \(\ce{Hg_2^2+}\)
Transition and inner transition metallic elements behave differently than primary group elements. Most transition element cations have 2+ or three+ charges that result from the loss of their outermost s electron(s) offset, sometimes followed by the loss of one or two d electrons from the next-to-outermost shell. For example, iron (1south 22s 22p vithreesouthward ii3p six3d 64south 2) forms the ion Fe2+ (1southward 22south 2iip sixthrees 23p vi3d 6) by the loss of the ivsouth electrons and the ion Fe3+ (onedue south 22s 22p 63s 23p 63d 5) by the loss of the 4s electrons and one of the threed electrons. Although the d orbitals of the transition elements are—according to the Aufbau principle—the last to make full when edifice up electron configurations, the outermost s electrons are the beginning to be lost when these atoms ionize. When the inner transition metals form ions, they ordinarily have a three+ accuse, resulting from the loss of their outermost due south electrons and a d or f electron.
Case \(\PageIndex{3}\): Determining the Electronic Structures of Cations
There are at least fourteen elements categorized as "essential trace elements" for the human trunk. They are called "essential" because they are required for healthy bodily functions, "trace" considering they are required simply in pocket-size amounts, and "elements" in spite of the fact that they are really ions. Two of these essential trace elements, chromium and zinc, are required as Cr3+ and Znii+. Write the electron configurations of these cations.
Solution
First, write the electron configuration for the neutral atoms:
- Zn: [Ar]3d 104s 2
- Cr: [Ar]threed 5fours one
Side by side, remove electrons from the highest energy orbital. For the transition metals, electrons are removed from the southward orbital first and so from the d orbital. For the p-block elements, electrons are removed from the p orbitals and then from the s orbital. Zinc is a member of group 12, and so it should have a charge of 2+, and thus loses only the two electrons in its southward orbital. Chromium is a transition chemical element and should lose its s electrons and and so its d electrons when forming a cation. Thus, we find the post-obit electron configurations of the ions:
- Zn2+: [Ar]3d 10
- Cr3+: [Ar]iiid 3
Exercise \(\PageIndex{iii}\)
Potassium and magnesium are required in our diet. Write the electron configurations of the ions expected from these elements.
- Answer
-
Thousand+: [Ar], Mgii+: [Ne]
Electronic Structures of Anions
Most monatomic anions grade when a neutral nonmetal atom gains enough electrons to completely fill up its outer due south and p orbitals, thereby reaching the electron configuration of the adjacent element of group 0. Thus, it is simple to determine the charge on such a negative ion: The charge is equal to the number of electrons that must be gained to fill up the s and p orbitals of the parent atom. Oxygen, for example, has the electron configuration anesouth ii2s 22p four, whereas the oxygen anion has the electron configuration of the noble gas neon (Ne), 1s 22south 22p vi. The two additional electrons required to fill up the valence orbitals give the oxide ion the accuse of 2– (Otwo–).
Case \(\PageIndex{iv}\): Determining the Electronic Structure of Anions
Selenium and iodine are two essential trace elements that grade anions. Write the electron configurations of the anions.
Solution
Se2–: [Ar]3d x4south ii4p six
I–: [Kr]4d x5southward ii5p half dozen
Exercise \(\PageIndex{4}\)
Write the electron configurations of a phosphorus atom and its negative ion. Give the charge on the anion.
- Answer
-
P: [Ne]threes 23p 3
P3–: [Ne]3s two3p vi
In ionic compounds, electrons are transferred between atoms of different elements to course ions. But this is not the but style that compounds can be formed. Atoms tin likewise brand chemic bonds by sharing electrons betwixt each other. Such bonds are called covalent bonds. Covalent bonds are formed between two atoms when both accept similar tendencies to attract electrons to themselves (i.due east., when both atoms have identical or fairly like ionization energies and electron affinities). For example, two hydrogen atoms bond covalently to form an Htwo molecule; each hydrogen atom in the H2 molecule has 2 electrons stabilizing information technology, giving each atom the aforementioned number of valence electrons every bit the element of group 0 He.
Compounds that contain covalent bonds showroom different concrete properties than ionic compounds. Considering the attraction between molecules, which are electrically neutral, is weaker than that between electrically charged ions, covalent compounds generally accept much lower melting and humid points than ionic compounds. In fact, many covalent compounds are liquids or gases at room temperature, and, in their solid states, they are typically much softer than ionic solids. Furthermore, whereas ionic compounds are skilful conductors of electricity when dissolved in water, most covalent compounds, being electrically neutral, are poor conductors of electricity in any country.
Summary
Video \(\PageIndex{2}\) : An overview of the part of orbitals in electron configurations and how to write electron configurations.
The relative energy of the subshells determine the order in which atomic orbitals are filled (1s, 2due south, iip, 3due south, 3p, 4s, 3d, 4p, and so on). Electron configurations and orbital diagrams tin be determined by applying the Pauli exclusion principle (no two electrons can have the same set of four quantum numbers) and Hund'southward rule (whenever possible, electrons retain unpaired spins in degenerate orbitals).
Electrons in the outermost orbitals, called valence electrons, are responsible for most of the chemic behavior of elements. In the periodic table, elements with coordinating valence electron configurations usually occur within the same group. There are some exceptions to the predicted filling order, peculiarly when half-filled or completely filled orbitals can be formed. The periodic tabular array can be divided into three categories based on the orbital in which the terminal electron to be added is placed: main group elements (s and p orbitals), transition elements (d orbitals), and inner transition elements (f orbitals).
Glossary
- Aufbau principle
- procedure in which the electron configuration of the elements is determined by "edifice" them in order of atomic numbers, adding 1 proton to the nucleus and one electron to the proper subshell at a time
- cadre electron
- electron in an atom that occupies the orbitals of the inner shells
- electron configuration
- electronic structure of an atom in its ground country given as a listing of the orbitals occupied by the electrons
- Hund'south rule
- every orbital in a subshell is singly occupied with one electron before any one orbital is doubly occupied, and all electrons in singly occupied orbitals have the same spin
- orbital diagram
- pictorial representation of the electron configuration showing each orbital equally a box and each electron equally an arrow
- valence electrons
- electrons in the outermost or valence shell (highest value of n) of a footing-state atom; determine how an element reacts
- valence shell
- outermost shell of electrons in a ground-country atom; for main group elements, the orbitals with the highest n level (south and p subshells) are in the valence shell, while for transition metals, the highest energy southward and d subshells make up the valence beat and for inner transition elements, the highest southward, d, and f subshells are included
Contributors
-
Paul Flowers (University of Northward Carolina - Pembroke), Klaus Theopold (University of Delaware) and Richard Langley (Stephen F. Austin Country Academy) with contributing authors.Textbook content produced past OpenStax College is licensed under a Artistic Commons Attribution License iv.0 license. Download for free at http://cnx.org/contents/85abf193-2bd...a7ac8df6@nine.110).
- Adelaide Clark, Oregon Institute of Technology
- Crash Form Chemistry: Crash Course is a partition of Complexly and videos are free to stream for educational purposes.
Feedback
Accept feedback to requite almost this text? Click here.
Found a typo and want extra credit? Click hither.
taylorfingrifuread.blogspot.com
Source: https://chem.libretexts.org/Courses/Oregon_Institute_of_Technology/OIT:_CHE_202_-_General_Chemistry_II/Unit_3:_Periodic_Patterns/3.1:_Electron_Configurations
ارسال یک نظر for "How Many Unpaired Electrons Are Present in a Ground-state Atom From the Alkaline Earth Metal Family"